Berbagai Fakta Menarik Tentang Penyakit Lupus di Dunia – Lupus merupakan penyakit inflamasi kronis yang terjadi akibat kinerja sistem imun tubuh yang salah. Jika sistem imun tubuh dalam keadaan normal, maka seharusnya dapat memberikan perlindungan kepada tubuh dari serangan infeksi bakteri maupun virus. Akan tetapi, orang dengan penyakit lupus sistem imun tubuhnya justru menyerang jaringan […]
Berbagai Fakta Menarik Tentang Penyakit Lupus di Dunia
Berbagai Fakta Menarik Tentang Penyakit Lupus di Dunia – Lupus merupakan penyakit inflamasi kronis yang terjadi akibat kinerja sistem imun tubuh yang salah. Jika sistem imun tubuh dalam keadaan normal, maka seharusnya dapat memberikan perlindungan kepada tubuh dari serangan infeksi bakteri maupun virus. Akan tetapi, orang dengan penyakit lupus sistem imun tubuhnya justru menyerang jaringan […]
Link Slot Gacor Terbaru: Rahasia Memenangkan Jackpot Besar!
Halo pembaca yang budiman! Apakah kamu salah satu penggemar slot online yang sering bermain tetapi masih merasa sulit untuk memenangkan jackpot besar? Jangan khawatir, karena di artikel ini kami akan memberikan rahasia tentang link slot gacor terbaru yang dapat membantu kamu meningkatkan peluangmu untuk meraih kemenangan besar. Jangan lewatkan artikel ini! Kami akan memberikan rahasia […]
Tanda-tanda Gejala Lupus pada Wanita
Tanda-tanda Gejala Lupus pada Wanita – Lupus adalah penyakit autoimun yang dapat menyebabkan nyeri dan peradangan di seluruh bagian tubuh. Meskipun pria dapat mengembangkan penyakit ini, penyakit ini jauh lebih sering terjadi pada wanita. Faktanya, 90% dari semua kasus yang didiagnosis terjadi pada wanita. Namun sulit untuk mendiagnosisnya, karena gejala lupus pada wanita dapat sangat […]
Cara Mengenali Tanda-Tanda Umum Penyakit Lupus
Cara Mengenali Tanda-Tanda Umum Penyakit Lupus – Lupus bisa sulit untuk didiagnosis karena gejalanya bervariasi dari orang ke orang. Anda harus berkonsultasi dengan penyedia layanan kesehatan jika Anda merasa memiliki tanda-tanda umum penyakit tersebut. Cara Mengenali Tanda-Tanda Umum Penyakit Lupus lupusmn – Lupus adalah penyakit autoimun yang menyerang berbagai bagian tubuh. Systemic lupus erythematosus (SLE) […]
Bagaimana Lupus Dapat Mempengaruhi Tubuh
Bagaimana Lupus Dapat Mempengaruhi Tubuh – Pada penderita lupus, sistem Imun kemudian akan melakukan adaptasi pengenalan selanjutnya akan menyerang jaringan tubuh. Peristiwa seperti ini mirip dengan “friendly fire” dan menyebabkan peradangan di berbagai bagian tubuh. Bagaimana Lupus Dapat Mempengaruhi Tubuh lupusmn – Namun, penting untuk disadari bahwa lupus dapat mempengaruhi orang yang berbeda dengan cara yang […]
Apakah Lupus Berakibat Mematikan?
Apakah Lupus Berakibat Mematikan? – Dalam kebanyakan kasus, lupus tidak berakibat fatal. Faktanya, 80% hingga 90% orang yang memiliki penyakit autoimun ini kemungkinan besar akan hidup normal. Apakah Lupus Berakibat Mematikan? lupusmn – Namun, beberapa orang meninggal karena penyakit tersebut, di mana sistem kekebalan menyerang organ dan jaringan tubuh Anda. Apakah Lupus Ada Obatnya? Belum, meskipun […]
Panduan Bagaimana Cara Mengatasi lupus
Panduan Bagaimana Cara Mengatasi lupus – Memiliki lupus dapat membuat kehidupan sehari-hari menjadi menantang. Ketika lupus Anda aktif, gejala seperti kekakuan sendi, nyeri, kelelahan, kebingungan, atau depresi dapat membuat tugas-tugas sederhana menjadi sulit dan terkadang tidak mungkin dilakukan. Karena gejala ini tidak terlihat, orang-orang di sekitar Anda mungkin kesulitan memahami perasaan Anda. Panduan Bagaimana Cara Mengatasi […]
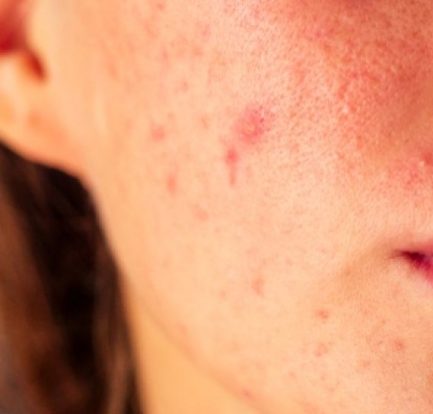
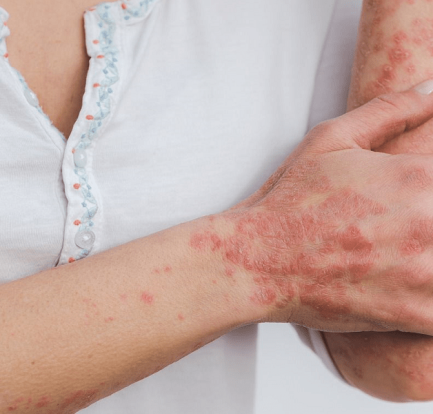
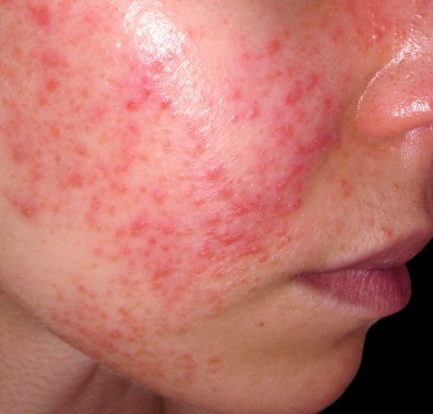
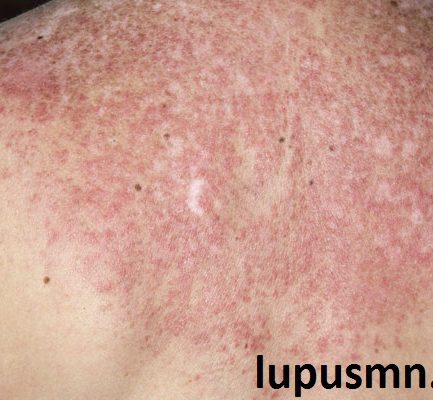
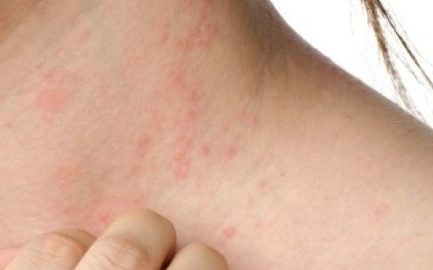
Penyakit Kulit dan Masalah Kulit Spesifik Lupus
Penyakit Kulit dan Masalah Kulit Spesifik Lupus – Kebanyakan orang dengan lupus mengalami semacam keterlibatan kulit selama perjalanan penyakit mereka. Faktanya, kondisi kulit terdiri dari 4 dari 11 kriteria yang digunakan oleh American College of Rheumatology untuk mengklasifikasikan lupus. Penyakit Kulit dan Masalah Kulit Spesifik Lupus lupusmn – Ada tiga jenis utama penyakit kulit khusus untuk […]
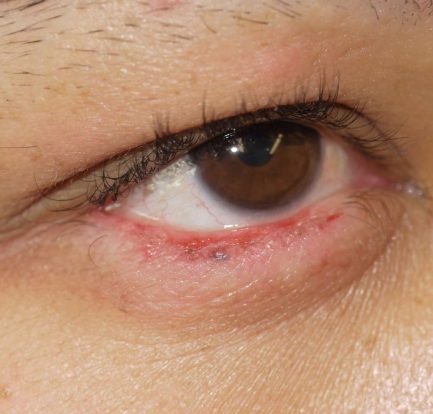
Apa Yang Perlu Diketahui Tentang Lupus Dan Masalah Penglihatan
Apa Yang Perlu Diketahui Tentang Lupus Dan Masalah Penglihatan – Lupus dapat menyebabkan mata kering, peradangan, lesi pada retina dan kelopak mata, serta kerusakan saraf. Perubahan ini dapat meningkatkan risiko masalah penglihatan, termasuk kehilangan penglihatan. Apa Yang Perlu Diketahui Tentang Lupus Dan Masalah Penglihatan lupusmn – Lupus adalah kondisi autoimun kompleks yang menyerang sekitar 1,5 […]
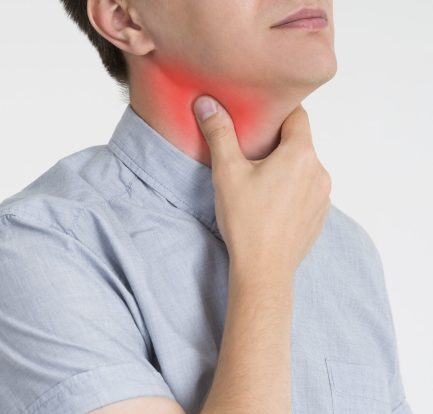
Apa Hubungan Antara Lupus Dan Hipertiroidisme?
Apa Hubungan Antara Lupus Dan Hipertiroidisme? – Lupus adalah kondisi autoimun jangka panjang yang menyebabkan peradangan berbagai organ dalam tubuh. Ini juga dapat mempengaruhi tiroid, menyebabkan hipertiroidisme. Apa Hubungan Antara Lupus Dan Hipertiroidisme? lupusmn – Seseorang dapat menderita lupus dan hipertiroidisme pada waktu yang bersamaan. Artikel ini mengeksplorasi hubungan antara lupus dan hipertiroidisme, termasuk gejala, […]
Fakta-fakta Tentang Penyakit Lupus
Fakta-fakta Tentang Penyakit Lupus – Lupus adalah penyakit autoimun yang tersebar luas dan kronis (seumur hidup) yang, untuk alasan yang tidak diketahui, menyebabkan sistem kekebalan menyerang jaringan dan organ tubuh sendiri, termasuk sendi, ginjal, jantung, paru-paru, otak, darah, atau kulit. Fakta-fakta Tentang Penyakit Lupus lupusmn – Sistem kekebalan biasanya melindungi tubuh terhadap virus, bakteri, dan […]
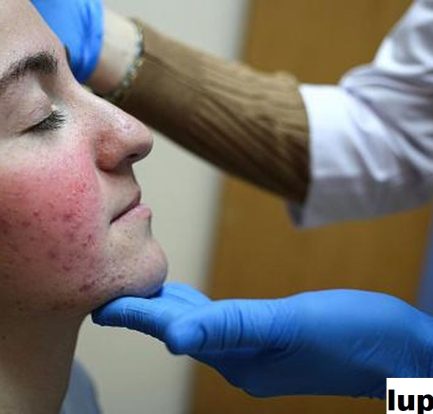
Bagaimana Cara Mendiagnosis Dan Mengobati Lupus?
Bagaimana Cara Mendiagnosis Dan Mengobati Lupus? – Bicaralah dengan dokter Anda jika Anda memiliki gejala lupus. Lupus adalah penyakit kronis yang tidak ada obatnya. Ini berarti Anda dapat mengatasinya dengan pengobatan, tetapi tidak akan hilang. Perawatan dapat membantu memperbaiki gejala Anda, mencegah kekambuhan, dan mencegah masalah kesehatan lain yang sering disebabkan oleh lupus. Perawatan Anda […]
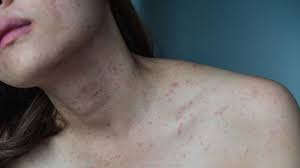
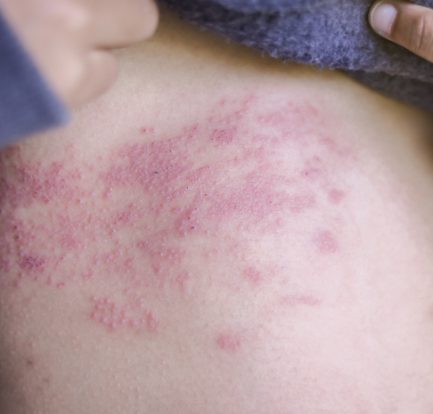
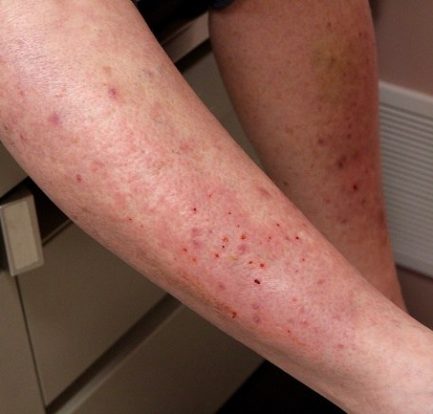
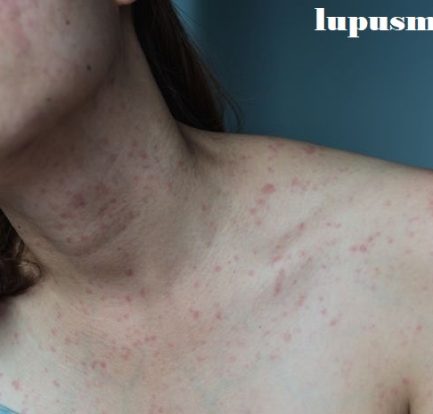
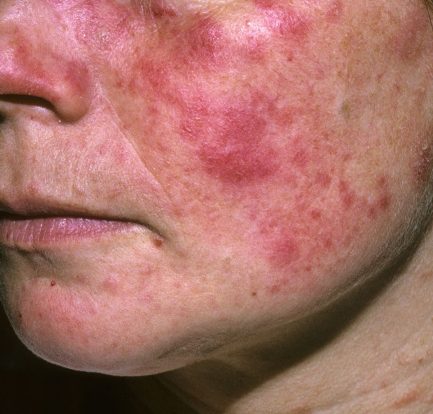
Ruam Kaki dan Lupus: Penyebab dan Pengobatan
Ruam Kaki dan Lupus: Penyebab dan Pengobatan – Sekitar dua dari tiga orang dengan lupus eritematosus sistemik (SLE) mengembangkan lupus kulit. SLE adalah jenis lupus yang paling umum dan mempengaruhi banyak organ. Namun, tidak semua penderita lupus (cutaneous lupus erythematosus) mengidap SLE. Namun, orang dengan salah satu tipe (atau keduanya) dapat mengalami ruam, termasuk ruam […]
Bagaimana Cara Untuk Mengatasi Penyakit Lupus
Bagaimana Cara Untuk Mengatasi Penyakit Lupus – Belajar untuk hidup dengan kelelahan terus-menerus dan rasa sakit kronis yang terkait dengan lupus kemungkinan besar akan berdampak pada pikiran dan tubuh Anda, sering kali menyebabkan frustrasi dan keputusasaan. Jadi kami telah mengumpulkan beberapa tip berguna untuk mengobati lupus. Hidup dengan rasa sakit kronis dapat membuat Anda merasa […]
8 Kemungkinan Pemicu Penyakit Lupus Autoimun
8 Kemungkinan Pemicu Penyakit Lupus Autoimun – Sulit dipercaya bahwa suatu penyakit dapat menyerang bagian tubuh mana pun, namun sebagian besar tetap tersembunyi dari mata telanjang. Tapi itulah kenyataan bagi hampir 1,5 juta orang Amerika yang hidup dengan beberapa bentuk lupus. Lupus adalah penyakit autoimun, artinya sistem kekebalan tubuh manusia mulai menyerang sel dan jaringan […]
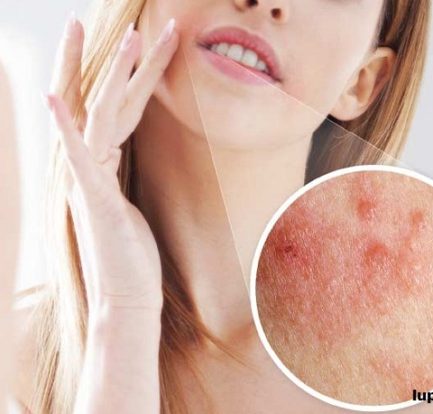
Penyebab Penyakit Lupus, Ciri-Ciri Dan Gejalanya
Penyebab Penyakit Lupus, Ciri-Ciri Dan Gejalanya – Bukan, itu bukan tokoh fiktif yang serialnya marak di tahun 1990-an, melainkan nama sebuah penyakit. Kondisi yang jarang terdengar hingga saat ini menjadi perbincangan karena Selena Gomez disebut-sebut mengalami kondisi tersebut. Menurut medicalnewstoday.com, lupus adalah penyakit autoimun jangka panjang yang menyebabkan sistem kekebalan tubuh menjadi hiperaktif. Penyebab Penyakit […]
Penyakit Lupus Eritematosus Sistemik Apa Penyebabnya
Penyakit Lupus Eritematosus Sistemik Apa Penyebabnya – Lupus berarti coyote dalam bahasa Latin. Penyakit kulit wajah ini awalnya diduga disebabkan oleh gigitan coyote. Namun seiring berjalannya waktu, ternyata penyakit lupus tidak hanya menyerang kulit wajah saja, tetapi juga bisa menyerang hampir setiap organ tubuh. Eritematosus berarti kemerahan sedangkan sistemik berarti menyebar ke berbagai organ tubuh. […]
Penyakit Lupus dan Beberapa Pengaruhnya Pada Wanita
Penyakit Lupus dan Beberapa Pengaruhnya Pada Wanita – Lupus adalah penyakit autoimun kronis yang menyerang lebih banyak wanita daripada pria. Jika Anda menderita lupus, Anda berisiko lebih tinggi mengalami masalah kesehatan lain yang umum terjadi pada wanita, seperti penyakit jantung dan osteoporosis. Lupus adalah penyakit autoimun kronis (seumur hidup) yang dapat menyerang bagian tubuh manapun. […]
Semua yang Perlu Anda Ketahui Tentang Lupus
Semua yang Perlu Anda Ketahui Tentang Lupus – Lupus adalah penyakit autoimun kronis yang dapat menyebabkan peradangan di seluruh tubuh. Namun, karena terutama merupakan kondisi lokal, tidak selalu sistemik. Penyakit autoimun adalah kondisi yang menyebabkan sistem kekebalan tubuh meradang dan menghancurkan sel-selnya sendiri. Banyak penderita lupus mengalami gejala ringan yang bisa menjadi parah tanpa pengobatan […]
Edukasi pasien: Systemic lupus erythematosus (Beyond the Basics)
Edukasi pasien: Systemic lupus erythematosus (Beyond the Basics) – Lupus eritematosus sistemik (juga disebut SLE atau hanya “lupus”) adalah penyakit kronis yang mempengaruhi banyak bagian tubuh. Lupus adalah penyakit autoimun, mengacu pada sistem kekebalan tubuh, yang “biasanya melindungi tubuh dari infeksi.” Ia menyerang jaringannya sendiri seolah-olah itu adalah benda asing. Hal ini dapat menyebabkan rasa […]
Kaitan Microbiome Pada Penyakit Autoimun
Kaitan Microbiome Pada Penyakit Autoimun – Dalam beberapa tahun terakhir, penyakit autoimun mulai dikenal di masyarakat dengan munculnya beberapa selebriti yang berbagi pengalamannya dengan penyakit autoimun. Dari Selena Gomez, Kim Kardashian, Laditya Dika, Cornelia Agatha hingga Ashanti, mereka membagikan kondisi mereka di media sosial dan di outlet berita untuk membantu lebih banyak orang mengetahui kondisi […]
Peran Akupunktur Pada Penyakit Lupus SLE
Peran Akupunktur Pada Penyakit Lupus SLE – SLE (systemic lupus erythematosus) adalah penyakit autoimun kronis yang disebabkan oleh peradangan pada jaringan dan organ tubuh seperti kulit, darah, persendian, ginjal, dan otak. Dalam keadaan normal, sistem kekebalan tubuh melindungi terhadap infeksi, tetapi ketika sistem kekebalan terganggu, tubuh dapat menyerang jaringannya sendiri. ) mampu mengidentifikasi Antigen dapat […]
SLE (Systemic Lupus Erythematosus) : Gejala
SLE (Systemic Lupus Erythematosus) : Gejala – Lupus eritematosus sistemik, atau biasa disingkat SLE, adalah jenis penyakit lupus yang menyebabkan peradangan pada hampir setiap organ tubuh, termasuk persendian, kulit, paru-paru, jantung, pembuluh darah, ginjal, sistem saraf, dan sel darah. SLE adalah jenis lupus yang dialami kebanyakan orang. Kebanyakan orang dengan SLE dapat menjalani kehidupan normal […]
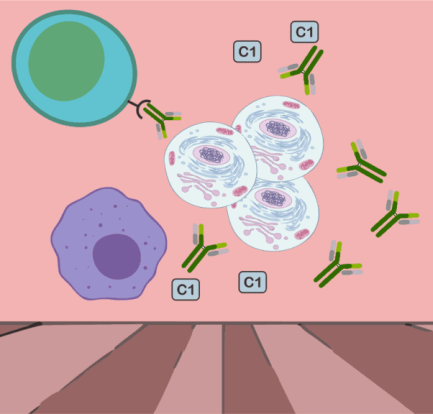
Patogenesis dan Patofisiologi SLE Penyakit Lupus
Patogenesis dan Patofisiologi SLE Penyakit Lupus – Memahami etiologi SLE dan patofisiologi SLE sangat penting untuk pengobatan yang tepat dari penyakit ini. Seperti yang Anda ketahui, SLE atau lupus eritematosus sistemik adalah penyakit autoimun yang ditandai dengan hilangnya toleransi sistem imun terhadap komponen self-antigen akibat kerusakan autoantibodi pada jaringan dan organ tubuh. Patogenesis dan Patofisiologi […]
Gejala dan Manajemen Penyakit Lupus Sebagai Penyakit Autoimun
lupusmn – Lupus adalah sebuah penyakit autoimun secara sistemik yang dapat terjadi ketika suatu sistem kekebalan didalam tubuh dapat menyerang jaringan dan juga organ di sekitarnya sehingga menyebabkan peradangan. Faktanya, selama ini lupus lebih banyak menyerang wanita daripada pria. Kejadian lupus juga umum di antara orang Asia yang memiliki kulit gelap dan menggunakan kontrasepsi oral. […]
Mengenal Genetika Lupus Foundation of Minnesota
lupusmn – Lupus Foundation Genetics disebut dengan cara yang berbeda. Ini adalah “penyakit wanita”. Ini adalah penyakit keluarga. Referensi ini menunjukkan salah satu faktor penting tentang lupus lebih akurat. Penyakit ini tampaknya memiliki bagian drop. Mengenal Genetika Lupus Foundation of Minnesota – Ada lebih banyak pertanyaan karena bertaruh pada para profesional medis genetika untuk mendukung […]
Yang Harus anda Ketahui Gejala Lupus Minnesota
lupusmn – Ketika Anda menderita lupus, sesuatu terjadi dalam sistem pertahanan alami tubuh Anda (sistem kekebalan Anda) buat membuatnya bekerja secara nir sahih. Bukan hanya menargetkan hal-hal jelek misalnya virus & bakteri, namun pula menyerang sel & jaringan sehat. Ada beberapa jenis lupus, & setiap masalah berbeda. Gejala Yang Harus anda Ketahui Gejala Lupus Minnesota […]
Ciri-Ciri Penyakit Lupus
lupusmn – Lupus Foundation of Minnesota adalah keliru satu Yayasan yg poly menangani pasian penderita penyakit lupus pada Minnesota. Penyakit lupus adalah keliru satu penyakit yg sporadis sekali didengar sang masyarakat. Ciri-Ciri Penyakit Lupus – Padahal penyakit ini poly poly diderita & jua bisa menyerang siapa saja. Penyakit lupus sendiri adalah inflamasi kronis yg penyakitnya […]